The Biomarker Company
SphingoTec develops and markets innovative in vitro diagnostics solutions for novel and proprietary blood-based protein biomarkers. Our aspiration is to effectively translate scientific advancements into routine clinical practice. We develop biomarkers addressing diagnostically underserved critical care conditions such as acute kidney injury, sepsis, and acute heart failure. First and foremost, our portfolio of tests supports better management of critically ill patients, where fast and reliable information is of the essence in taking treatment decisions.
Our Mission
To improve patient outcomes with innovative diagnostic solutions for acute and critical care.
Actionable insights in real-time.
Certified Quality
SphingoTec is committed to providing products that meet and exceed customer and regulatory requirements.
We produce our products in accordance with EN ISO 13485:2016.
ISO 13485 Certificate (English) ISO 13485 Certificate (German)
Innovative biomarkers
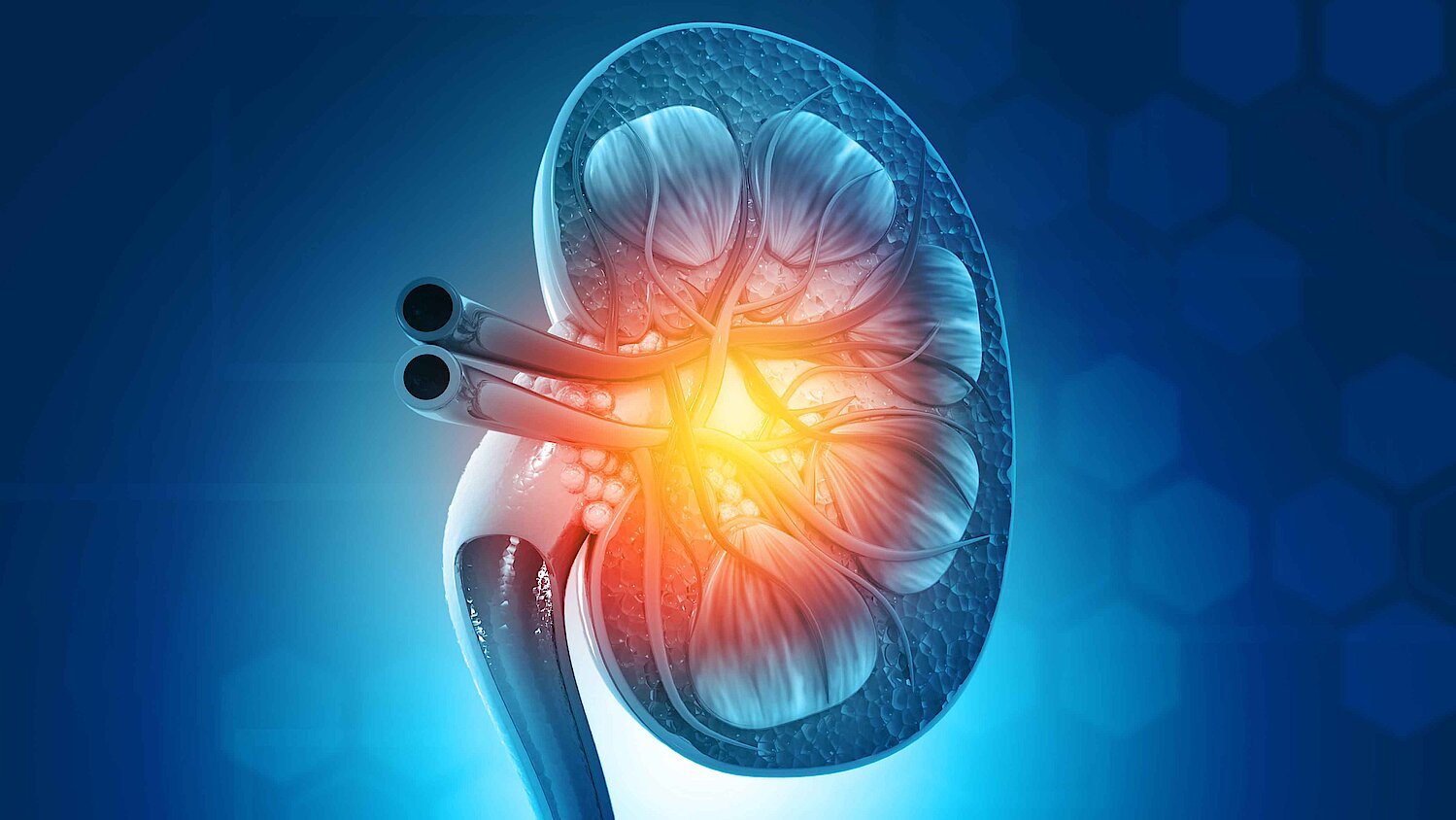
Kidney function biomarker
Proenkephalin A 119-159 (penKid)
The limitations of the current standard of care diagnostics for acute kidney injury are well known, thus is the need to implement new biomarkers to assist better management of acute kidney injury. Timely information on kidney function is crucial to early initiate and adapt renal replacement therapy and nephro-protective strategies

Headquarter in Hennigsdorf, Germany
SphingoTec is a privately held company with a long history of biomarker discovery, development, and validation. We are the legal manufacturer of CE IVD microtiter plate (MTP) assays. Committed to providing solutions that meet and exceed customer and regulatory requirements, we manufacture our products in accordance with EN ISO 13485:2016.
Diagnostic platforms
Microtiter plate assay
The innovative biomarkers are available as CE IVD microtiter plate (MTP) assays. The MTP is a standard tool in analytical research and laboratories. It is a high throughput solution, allowing the measurement of 41 patient samples at once. SphingoTec’s MTP assays are based on a chemiluminescence sandwich immunoassay technology.
History
2023
SphingoTec receives the certificate being compliant with IVDR (EU) 2017/746.
2022
First licensing agreement for penKid closed with Boditech Med, Inc.
2022
SphingoTec is ISO 13485:2016 certified.
2020
The novel biomarkers are introduced in routine measurements in European university hospitals.
2020
Over 80,000 patients have been measured with our biomarker tests.
2018
SphingoTec enters market by deploying the novel biomarkers via a point-of-care solution and distribution network.
2018
Financing round: 25 million euro growth capital. Investors: HBM, Wellington Partners, and ILB.
2016
CE-IVD microtiter plate tests for penKid and bio-ADM launched.
2011 - 2013
Bio-ADM and penKid product development. First clinical validation.